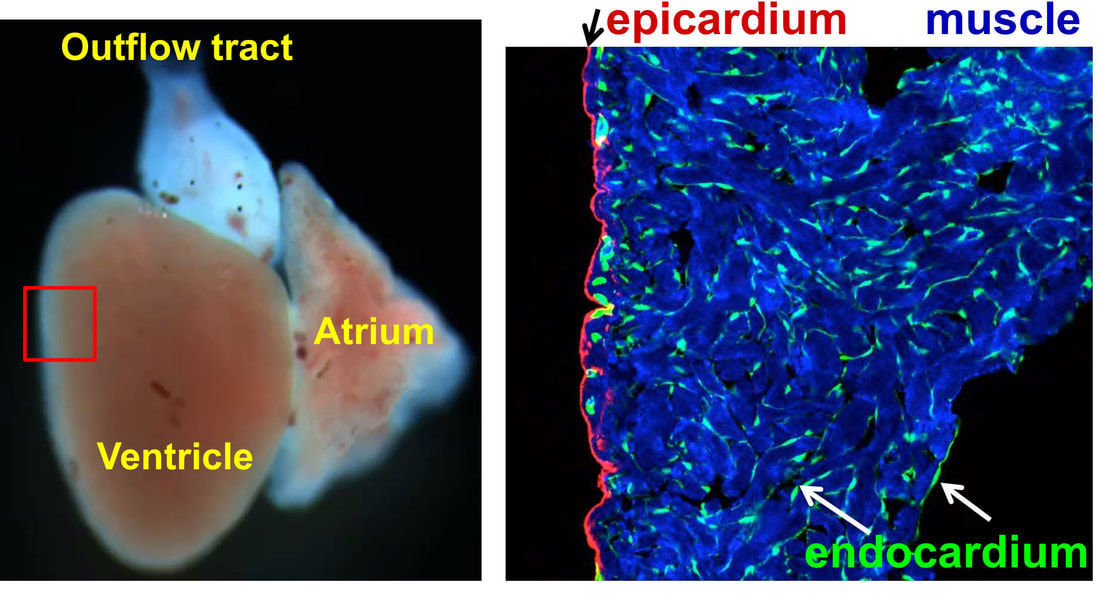
Heart Regeneration in zebrafish
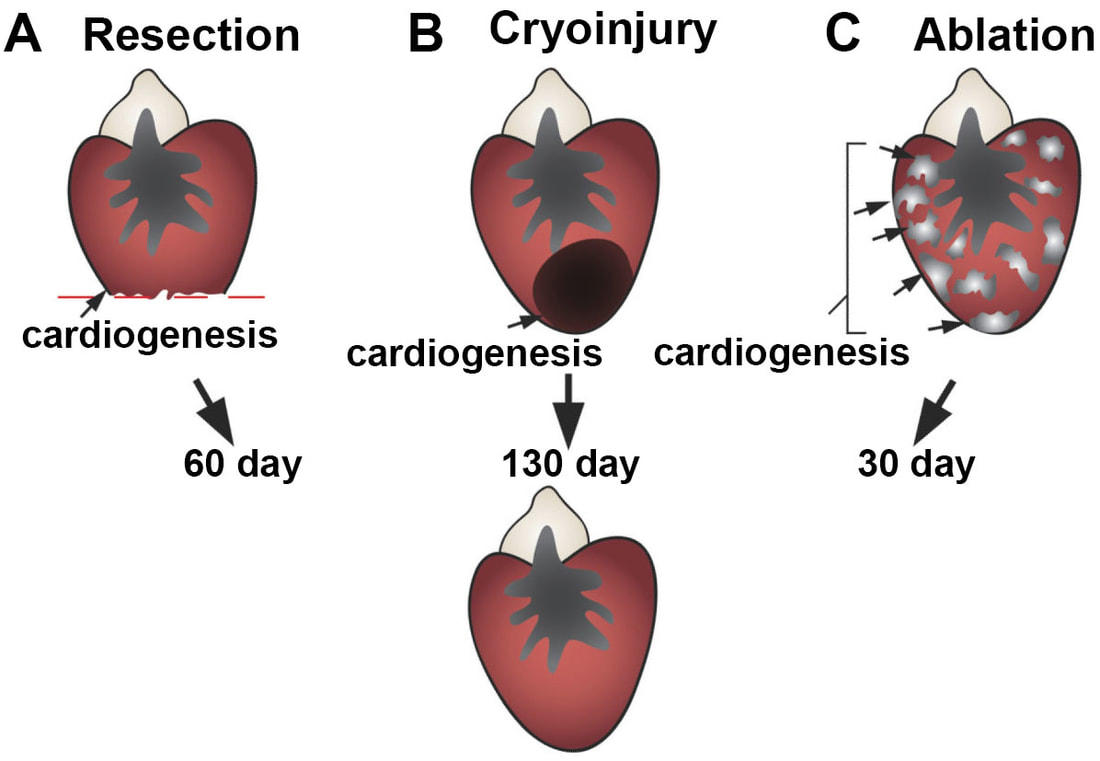
The adult mammalian (including human) hearts shows minimal regeneration of new cardiac muscle after ischemic myocardial infarction (MI). Unlike mammals, adult zebrafish possess a remarkable capacity for cardiac regeneration with minimal scarring after injuries removing up to 60% of CMs. Defining regenerative mechanisms in zebrafish will help shape strategies for mammalian heart repair. Zebrafish heart regeneration is achieved through the proliferation of existing CMs, which are quiescent without injury (as in mammalian CMs). CM proliferation is aided by cellular contributions and paracrine effects of non-muscle tissues, such as the epicardium and endocardium.
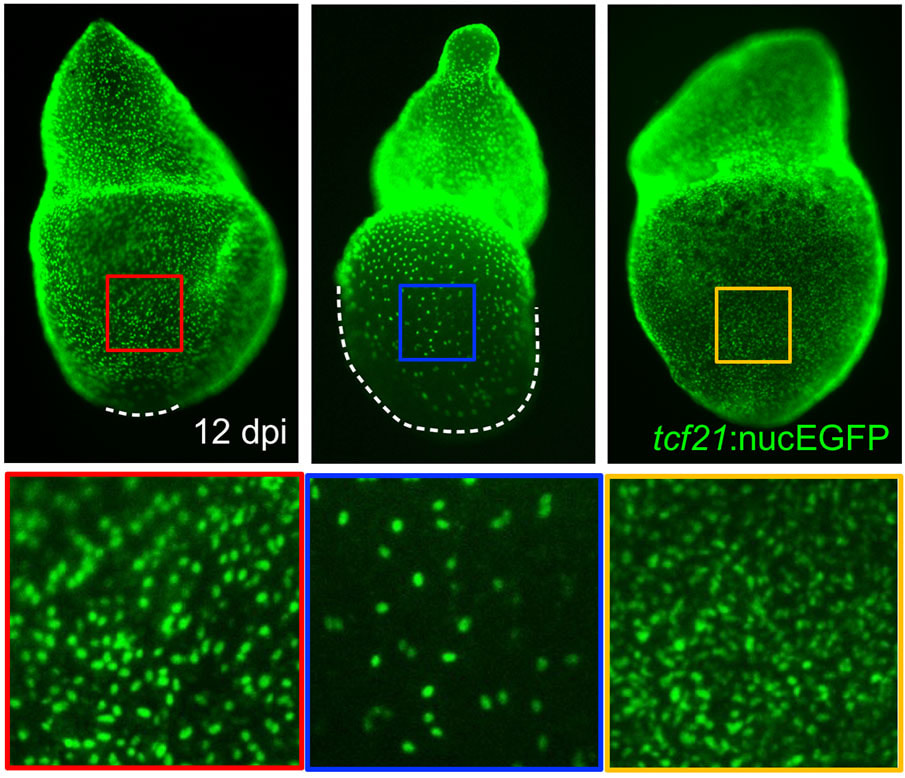
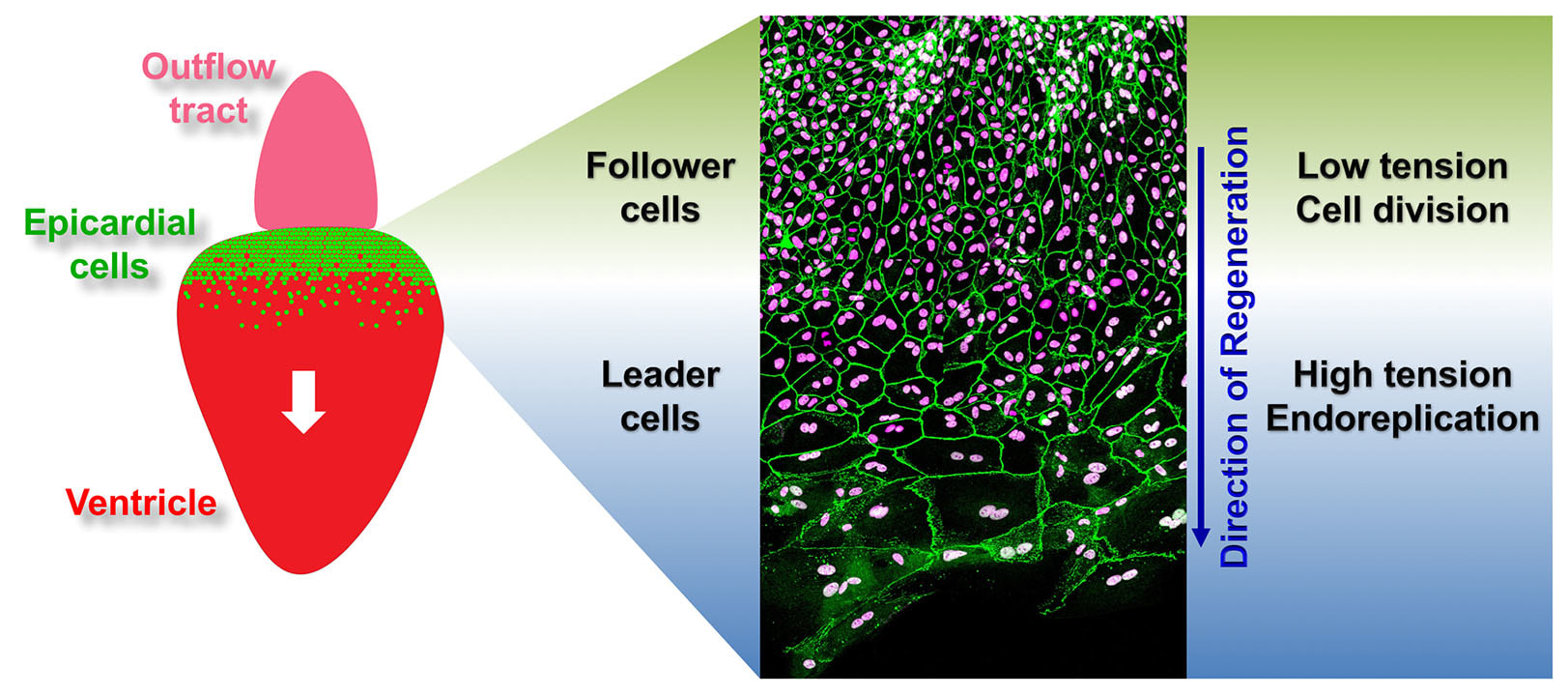
The epicardium enveloping the adult heart is a multipotent cardiac progenitor cell population, which is activated after heart injury (expressing developmental genes), proliferates, and supplies paracrine signals and other cell types during cardiac repair or regeneration in both zebrafish and mice. In a previous study, my colleagues and I found that an intact epicardium is required for CM regeneration in zebrafish (Wang and Cao et al., 2015, Nature). This suggests that enhancing the epicardial injury response would benefit CM regeneration. However, how epicardial cells respond to heart injury and further exert effects on muscle regeneration are poorly understood, and my recent finding of the cellular heterogeneity of the zebrafish epicardial cells added complexity to this question (Cao et al., 2016, Development). This deficiency of knowledge represents a major barrier for harnessing epicardium for therapeutic goals. To address this, using a combination of zebrafish model, explant tissue culture, high-throughput sequencing, chemical screening, live imaging, and genome editing approaches (such as CRISPR/Cas9), we will dissect how epicardial cell activation, proliferation, and lineage specification are molecularly and genetically regulated to engage in heart muscle regeneration.
The epicardium enveloping the adult heart is a multipotent cardiac progenitor cell population, which is activated after heart injury (expressing developmental genes), proliferates, and supplies paracrine signals and other cell types during cardiac repair or regeneration in both zebrafish and mice. In a previous study, my colleagues and I found that an intact epicardium is required for CM regeneration in zebrafish (Wang and Cao et al., 2015, Nature). This suggests that enhancing the epicardial injury response would benefit CM regeneration. However, how epicardial cells respond to heart injury and further exert effects on muscle regeneration are poorly understood, and my recent finding of the cellular heterogeneity of the zebrafish epicardial cells added complexity to this question (Cao et al., 2016, Development). This deficiency of knowledge represents a major barrier for harnessing epicardium for therapeutic goals. To address this, using a combination of zebrafish model, explant tissue culture, high-throughput sequencing, chemical screening, live imaging, and genome editing approaches (such as CRISPR/Cas9), we will dissect how epicardial cell activation, proliferation, and lineage specification are molecularly and genetically regulated to engage in heart muscle regeneration.